New Preprint: THR-6E for Risk Stratification in ER+/HER2− Breast Cancer
We are excited to share our new preprint, “THR-6E: A Six-Gene Cell-of-Origin Signature Stratifies Risk and Predicts Systemic Therapy Response in ER+/HER2− Breast Cancer,” now available on medRxiv.
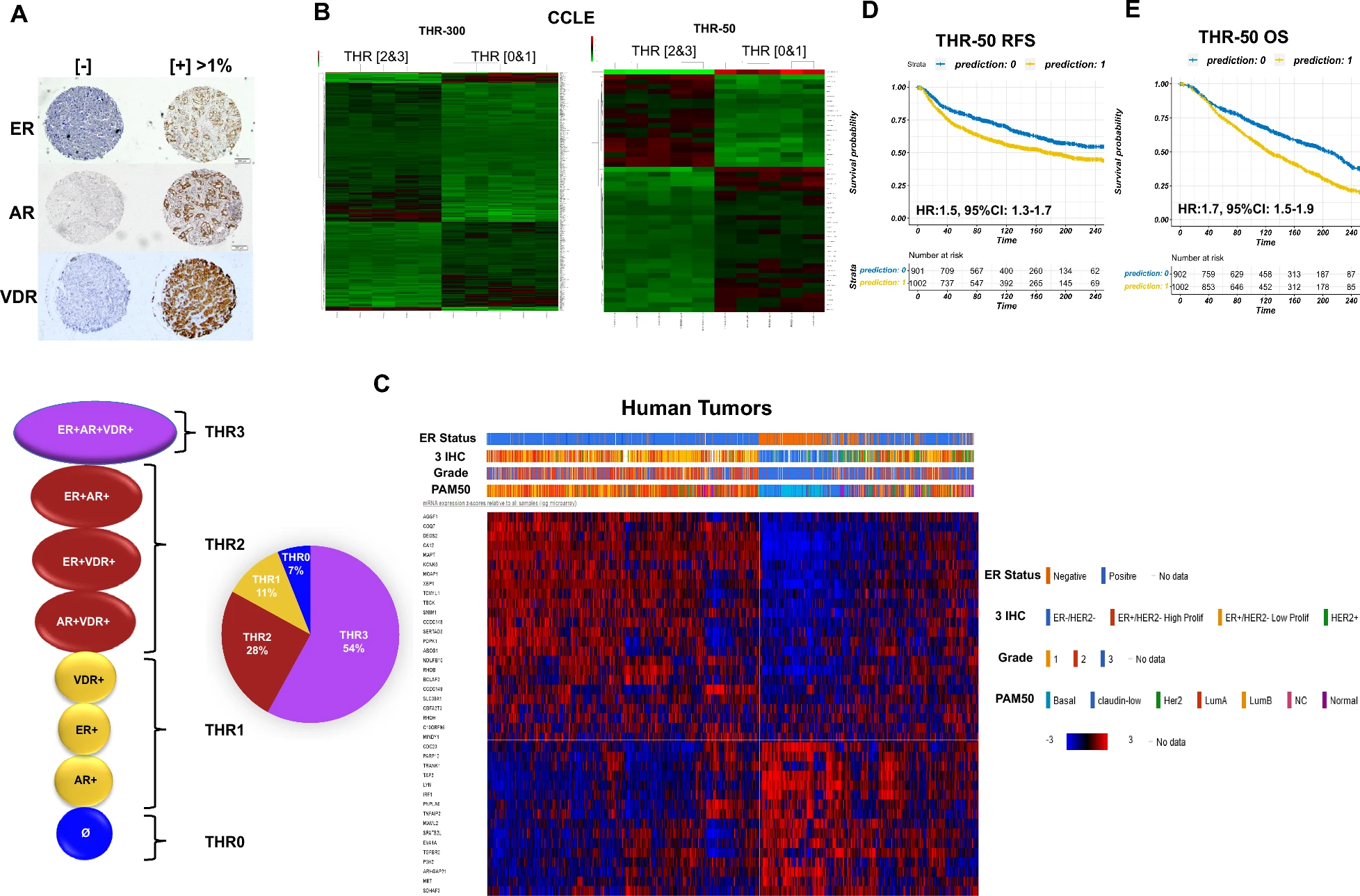
Building on our earlier work on the triple hormone receptor (THR) signature, THR-6E is a compact six-gene cell-of-origin signature (KIF4A, KIF2C, CDC20, FAM64A, TPX2, LMNB2) that robustly stratifies relapse-free survival and predicts response to both endocrine therapy and chemotherapy in ER+/HER2− breast cancer. The signature was validated across >7,000 breast cancer cases from multiple independent cohorts, including the I-SPY2 adaptive clinical trial.
Notably, THR-6E maintains its prognostic and predictive value in lymph node-positive patients — a subgroup where existing genomic assays such as Oncotype DX, MammaPrint, and Prosigna have limited clinical utility and are not yet strongly recommended by current guidelines. THR-6E predicted chemotherapy response in node-positive ER+/HER2− disease with ~70% sensitivity and specificity across taxane-, anthracycline-, and FEC-based regimens, addressing a key unmet need in this patient population.